Describe How Elements Are Arranged in the Periodic Table
What did Mendeleev discover about the elements when they were arranged. All periods begin with an alkali metal in group 1 and end with a noble gas in group 188A.
What Do Elements In Group 2 Of The Periodic Table Have In Common Quora
Most of the work that was done to arrive at the periodic table that we know can be attributed to a Russian chemist named Dmitri Mendeleev.
. Each horizontal row on the periodic table is called a period. Solutions for Chapter 5 Problem 9CRQ. Rows called periods in order of increasing atomic number.
The elements in the Periodic Table are arranged in order of increasing atomic number. He arranged the elements in the grid of 18 columns and 7 rows. Which general area contains the nonmetallic elements.
Describe the periodic table of the elements. Elements are arranged in order of increasing atomic number. Elements are listed in numerical order by atomic number.
Elements are arranged from left to right and top to bottom in order of increasing atomic number. The Periodic table elements are arranged in the increasing order of their atomic number. Each vertical column on the.
Elements are arranged in order of increasing atomic number. Groups are read left to right and are organized by their electrons. The atomic number is the number of protons in an atom of that.
Elements in the periodic table are arranged in order of increasing atomic proton number. Rows called periods in order of increasing atomic number. In the modern periodic table the elements are arranged according to their atomic number not their relative atomic mass.
And it was named as Modern Periodic table. Which general area of the periodic table contains the metallic elements. They are placed with the lowest atomic number first and elements with increasing atomic numbers run to the right.
The elements are arranged into 18 groups columns and seven periods rows by increasing atomic number. Describe the difference between a period and a group. Compare metals nonmetals and metalloids based on their properties and on their location in the periodic table.
Each element has its own unique atomic number which is the number of protons in the nuclei of its atoms and which defines the element. 1869 - Two scientists determined a way to determine a way to put the elements in order it was Lothar Meyer and Dmitri Mendeleev. The rows are called periods.
The arrangement of elements in the Periodic table starts from the very first top left corner. Order generally coincides with increasing atomic mass. Chemistry a table of the elements arranged in order of increasing atomic number based on the periodic law.
In all forms of todays modern periodic table elements are arranged in order of increasing atomic number. Each horizontal row of elements in the periodic table is a period. They indicate the number of electrons in the outer shell of an atom.
In 1913 Henry Moseley arranged the known elements in the increasing order of their Atomic Number. What were the contributions of Mendeleev to the periodic table. I can describe the connection between atomic number and the order of elements in the Periodic Table.
Arranging the Elements Objectives Describe how Mendeleev arranged elements in the first periodic table. This Periodic table consists of vertical columns called groups and horizontal rows called periods. The periodic table 51 The arrangement of the elements ESABM The periodic table of the elements is a method of showing the chemical elements in a table with the elements arranged in order of increasing atomic number.
In the periodic table the elements are arranged into. How are the elements are organized in the periodic table. At present there are three versions of the periodic table each with its own unique column headings in wide use.
He assembled the table by classifying chemical elements in an order based on their periodicity of chemical and physical properties. The first element with atomic number 1 ie hydrogen is placed in the first cell then gradually the elements with atomic number 2 3 4 upto 118 are placed from the left to right in. Each row from left to right is called a period each elements in those rows share similar electron configurations.
There are seven periods on the periodic table. Elements with the same number of electron shells are arranged in the horizontal rows periods and elements with similar properties are arranged in vertical columns groups. The periodic table of elements arranges all of the known chemical elements in an informative array.
Heres how it works. The chemical elements are arranged in order of increasing atomic number. Vertical columns called groups where the elements have similar properties.
Rows called periods in order of increasing atomic number vertical columns called groups where the elements have similar properties. They are also arranged in vertical columns known as groups with these groupings based on shared properties. The general structure of the grid is organized by rows and columns of elements this makes it easy to Identify and find elements on the periodic table.
In the periodic table the elements are arranged into. The horizontal rows are called periods and the vertical columns are called groups. Atomic number is unique to each element.
Atomic number and protons In the modern periodic table the elements are arranged according to their atomic number - not their relative atomic mass. How are the elements arranged in the table. Rows are called Periods.
What significance is there in the way the elements are arranged into vertical groups. Mendeleev found that when all the known chemical elements were arranged in order of increasing atomic weight the resulting table displayed a recurring pattern or periodicity of properties within groups of elements. In the periodic table the elements are arranged in columns and rows according to increasing atomic number see the table entitled Periodic Table.
Why is the periodic table a useful tool. In the modern periodic table elements are arranged by atomic number. 1864 - John Newlands published his own version of the periodic table and developed the Law of Octaves.
They indicate the number of shells energy levels in an atom. Elements in the same group have similar chemical properties because they have the same number of outer. Explain how elements are arranged in the modern periodic table.
Columns are called Groups. Each element has one more proton than the element preceding it. There are 18 vertical columns or groups in the standard periodic table.
Elements having similar chemical properties and electronic structures appear in vertical columns groups. Explain the meaning of atomic number of an elements in terms of position in the periodic table and number of protons in the nucleus. Vertical columns called groups where the elements have similar properties.
Atomic numbers clarified the arrangement of elements on the periodic table including Mendeleevs decision to place heavier tellurium ahead of iodine.
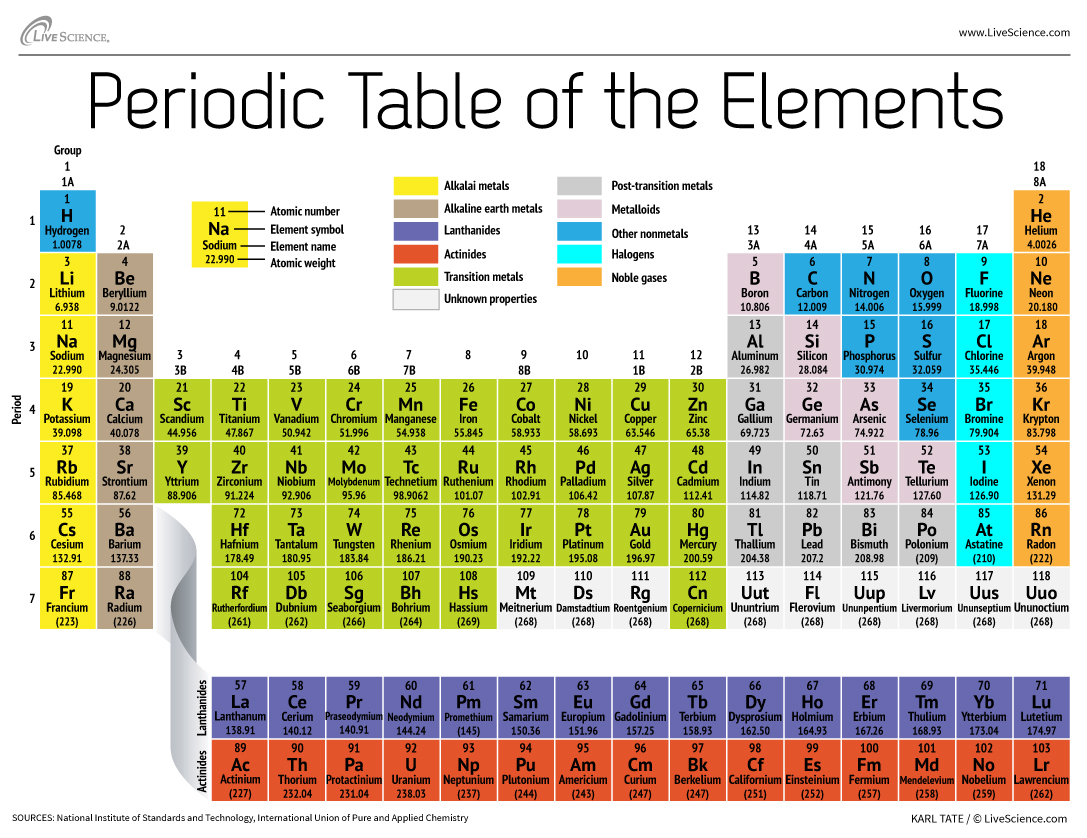
How The Periodic Table Groups The Elements Live Science

Describe The Arrangement Of Elements In The Periodic Table In Order Of Increasing Atomic Number Development Of The Periodic Table Johan Dobereiner Ppt Download

Periodic Table Of Elements With Names And Symbols Periodic Table Of The Elements Periodic Table Printable Periodic Table With Names
No comments for "Describe How Elements Are Arranged in the Periodic Table"
Post a Comment